Chapter contents
I. Introduction
Research into new drugs for hidradenitis suppurativa (HS) has greatly increased in the last 20 years. New drugs being studied in clinical trials may become good treatment options for helping treat HS in the future. This chapter explains the clinical trial process, what a drug target is, how HS occurs, and reviews the drugs being studied in clinical trials for HS (often called “the pipeline”).
II. What is a clinical trial?
Clinical trials test new drugs to see whether they are safe and effective for treating a given disease. There are four stages of clinical trials referred to as “phases”. Phase I trials test drug safety and look for harmful side effects. Phase II trials continue to evaluate safety and test whether a drug is effective at treating a specific disease, comparing people taking the drug being studied with people taking a “fake” drug (also called placebo group). Phase III trials test the effectiveness of drugs across larger and more diverse groups of people. In the United States, after the drug has been tested in Phase I through III trials, the drug may be approved by the Food and Drug Administration (FDA). Once a drug becomes approved by the FDA, doctors can prescribe the drug to patients. Phase IV trials take place after FDA approval and entail collecting more information as the drug is used by the larger general population. A detailed description of clinical trials Phases I-IV and a complete list of active clinical trials for HS can be found at clinicaltrials.gov.
III. What is a drug target?
Drugs work by binding and blocking “targets” that cause disease. The most common drug targets are called ligands and receptors. Ligands are small proteins that tell cells to carry out tasks. Ligands convey a message to the cell by binding to their specific receptor on the cell surface. Through ligands and receptors, cells can talk to each other and carry out functions. Problems with ligands or receptors can lead to cells carrying out the wrong functions that may lead to disease.
The immune system is a complex network of cells and proteins that defend our body against sickness and disease. When prompted, immune system cells release ligands called cytokines that bind receptors on other cells. As a result, cells are told to turn on (or activate) inflammation to fight off disease. Our current understanding is that the immune system is overactive in HS, causing inflammation when it is not needed and harming healthy tissue. Drugs used to treat HS can bind to and block cytokines or receptors involved in this process, stopping the inflammation and damage to healthy tissues that we see in HS.
There are many types of drugs, and a general principle is that the structure of each drug determines its overall function. One type of drug is called an antibody. Antibodies are special proteins made by the immune system that recognize and block harmful substances in the body, such as bacteria, viruses, and toxins. Antibodies can also be made in the laboratory and given as a drug to recognize and block disease-causing proteins. For instance, an antibody can be made to recognize and block a cytokine known to cause inflammation in HS. In this way, antibodies can be a good treatment for some patients with HS. Many drugs being made and studied for HS are antibodies and are given the ending to their name “-mab” (e.g., adalimumab). Most antibody drugs are given as an injection or an infusion.
IV. How does HS develop?
Knowing how HS occurs in the body helps researchers decide what targets may be important for new HS drugs to block. The red lumps (“nodules”), boils (“abscesses”), and tunnels that form in HS are thought to start with a plugged hair follicle that widens and bursts open into the skin. Abnormal hair follicle structure, hormones, smoking, increased body weight, and chronic rubbing of the skin may play a role in the hair follicle plugging and bursting. Abnormal levels of bacteria living on the skin and in the hair follicles may also add to the inflammation seen in HS.
Bursting of the hair follicle in the skin starts a series of events that cause the skin to become red, warm, swollen, and painful. The material of the ruptured hair follicle activates cells called macrophages and dendritic cells. These important cells act as the “guards” of the immune system and release cytokines that produce inflammation. some of these cytokines include tumor necrosis factor alpha (TNF-α) and interleukins (IL), such as IL-1, IL-17, and IL 36. One of the major effects of these cytokines is calling white blood cells called neutrophils to the skin. Neutrophils normally reside in the blood stream but can travel to the skin if they are needed to help fight infection or Neutrophils are the “first responder” cells and they both release toxic substances that may hurt tissues and call even more neutrophils into the skin. Pus forms as a result of neutrophils migrating to the skin and causing inflammation. Complement is another system used by the immune system to fight off infections. Proteins that are part of the complement system seem to be abnormally activated in HS. One protein involved in complement, called complement 5a (C5a), recruits even more neutrophils to the skin. The inflammation caused by neutrophils, cytokines, and complement lead to the nodules, abscesses, and draining tunnels seen in HS. Drugs that block the functions of neutrophils, cytokines, and complement may reduce the inflammation thought to cause HS.
V. Pipeline medical therapies
Drugs being studied in clinical trials for HS are described below based on the main target of each drug. Table 29.1 lists those clinical trials for HS that are active, recruiting, or not yet recruiting at clinicaltrials.gov as of January 1, 2023. Drugs being studied that do not yet have a formal name are typically identified by a combination of letters and numbers (e.g., ABC-123). Figure 1 illustrates targets of new drugs that are being studied for HS.
Neutrophils & Complement. As mentioned above, neutrophils are one of the cell types that are most active and destructive in HS. Large numbers of neutrophils are called to the skin by inflammatory cytokines and some fatty molecules, called lipids. An example of a lipid that recruits neutrophils to the skin is called LTB4. A new oral drug, called LYS006, stops cells from making LTB4 and therefore prevents neutrophils from migrating to the skin and causing inflammation. LYS006 is currently being studied for HS in a phase II trial.
Blocking the complement pathway can also reduce the number of neutrophils called to the skin. There are two drugs that have recently been studied for HS that block the complement pathway. BDB-001 is monoclonal antibody drug given as an injection that directly blocks C5a to lessen inflammation. The safety of this drug is currently being studied in early phase clinical trials. Avacopan, an oral C5a receptor blocker, and IFX-1 (also called vilobelimab), an injectable drug that blocks C5a itself, both recently completed phase II trials for HS. Press releases from both phase II studies have reported improvement in HS, but not significantly greater than placebo. However, makers of both Avacopan and IFX-1 are committed to further test whether these C5a blockers will be safe and effective for some HS patients.
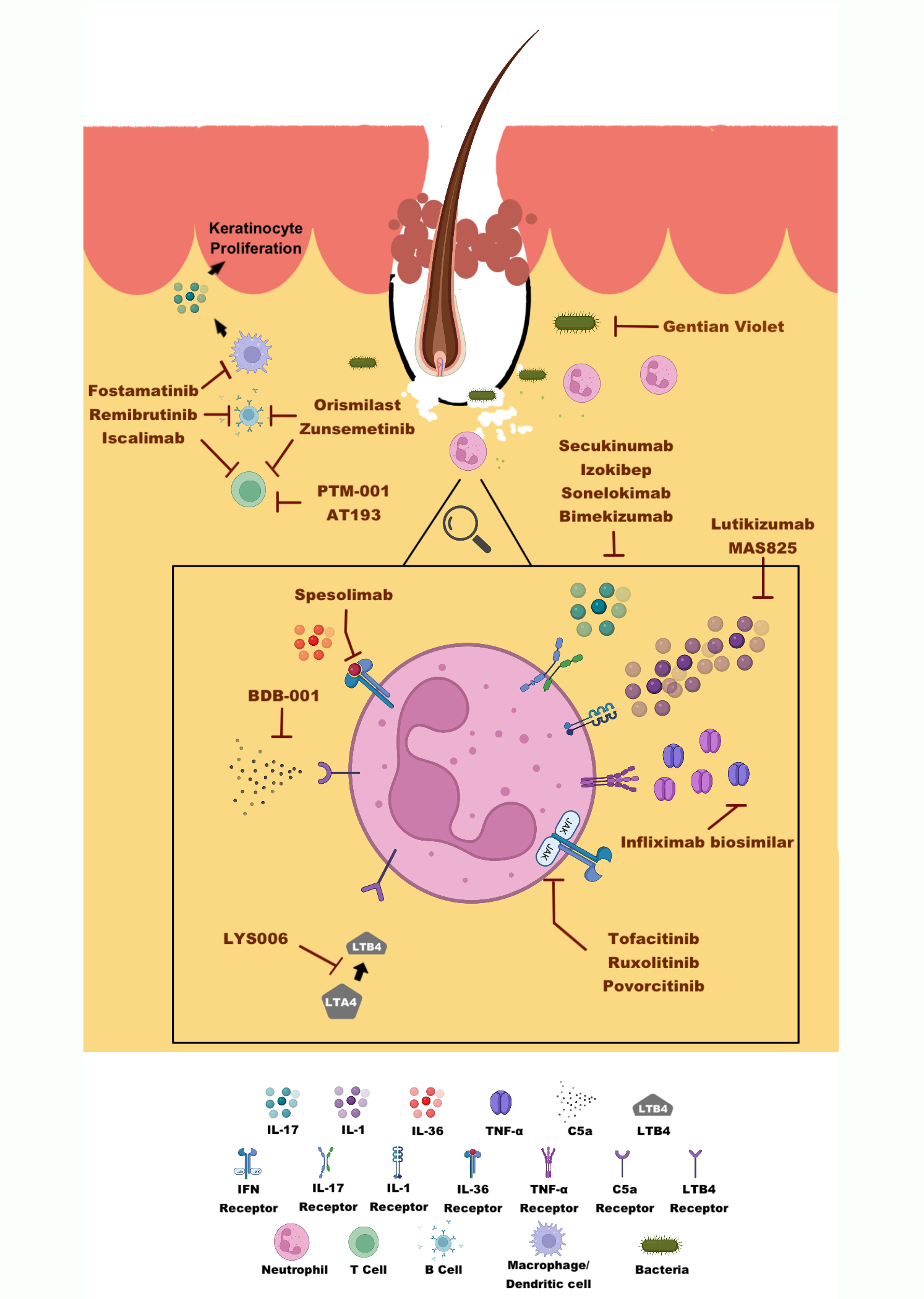
Cytokines. Adalimumab is an antibody drug that is currently the only FDA-approved drug treatment for moderate to severe HS. TNF-α levels are increased in HS and adalimumab blocks this cytokine from causing inflammation in the skin. Infliximab is another antibody drug that blocks TNF-α that is FDA-approved for rheumatoid arthritis, plaque psoriasis, and inflammatory bowel disease. A small study of 38 patients showed that treatment with infliximab for 8 weeks significantly improved HS compared to placebo. Because of these results and positive results from other small studies, infliximab is sometimes prescribed when HS fails to improve with adalimumab. Though not currently FDA-approved for HS, the North American clinical management guidelines for HS recommends infliximab for moderate to severe HS. A new drug that is similar to infliximab, sold under the name Infliximab biosimilar, has just entered the HS drug pipeline and is currently undergoing a phase I trial for HS.
Other cytokines involved in HS inflammation include IL-1, IL-17, and IL-36. There are several drugs in clinical trials that block these cytokines in attempt to stop the inflammation seen in HS.
IL-1 is a cytokine that causes inflammation and is found in high amounts in HS skin and blood. A small study showed that an IL-1 receptor blocker, Anakinra, was effective for HS. Since then, several new treatments aimed at blocking IL-1 have joined the medical pipeline for HS. Lutikizumab, a new antibody drug that binds IL-1 was recently developed and will be studied in an upcoming phase II trial for HS. MAS825, an IL-1 blocker that also blocks IL-18, another cytokine, has previously been studied for COVID-19 infections, and is now in a phase II trial investigating its efficacy for HS.
IL-17 is found in high amounts in HS skin and blood. One IL-17-blocking drug, called secukinumab, is FDA-approved for plaque psoriasis and psoriatic arthritis and has been in the drug pipeline for HS for several years. In Fall 2022, the makers of secukinumab shared the results of two large phase III trials. Excitingly, a significant number of patients treated with secukinumab in these trials experienced improvement in their HS compared with placebo. The makers of secukinumab plan to submit these results to the FDA in 2023 with hope that secukinumab can become the second FDA-approved medical therapy for HS. Bimekizumab, a unique IL-17 blocker showed very good results in a recent phase II trial. Bimekizumab also completed two phase III trials that showed greatly improved signs and symptoms of HS. The results of these studies have not yet been published, but results have been presented at national meetings and announced in press releases. Currently, the long-term effects are still being studied in a follow-up phase III trial seeing how patients do on the treatment over 120 weeks. Two additional new IL-17 blocking drugs similar to secukinumab, called izokibep and sonelokimab, will be also studied in upcoming phase II trials.
IL-36 is another cytokine that is found in high amounts in HS skin and is thought to contribute to the inflammatory process seen in HS. One anti body drug that blocks IL-36, called spesolimab, received FDA-approval for the treatment of a rare skin condition called generalized guttate psoriasis in October 2022. Spesolimab is currently being studied in a phase II trial for HS. Recently, another IL-36 blocker, Imsidolimab, completed its phase II trial for HS, and unfortunately, was not found to be effective.
Janus Kinase. Janus kinases (JAKs) are proteins attached to many cytokine receptors and help relay messages from ligands and receptors to the inside of the cell. There are four different JAK proteins (called JAK1-3 and TYK2) that are attached to many kinds of cytokine receptors. The first FDA-approved JAK blocker drugs were tofacitinib (JAK1-3 blocker) and ruxolitinib (JAK1-2 blocker). Tofacitinib is FDA-approved for treatment of rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis. So far, there have only been three reported cases of tofacitinib being effective for HS. Tofacitinib will be studied in a planned phase II trial for multiple inflammatory conditions in patients with Down Syndrome, including HS. Oral ruxolitinib is FDA-approved for treatment of several bone marrow diseases. Ruxolitinib is also made as a topical medicine. Topical ruxolitinib is FDA-approved for atopic dermatitis and vitiligo and will be studied in two phase II trials for HS. Povorcitinib, a new JAK1 blocker, has finished two small phase II trials and was shown to be safe and effective for treating HS in a small number of patients; a phase III trial is now underway.
Multiple immune system cells. Several of the new drugs in phase I and II trials block proteins and receptors that affect many different immune cells and pathways of inflammation. Fostamatanib, Remibrutinib, Zunsemetinib, and Orismilast, are all oral medications that affect the ability of various immune cells to function. Fostamatanib and Remibrutinib uniquely also affect B-cells, which produce antibodies. Zunsemetinib and Orismilast reduce the ability of immune cells to produce and respond to cytokines, specifically TNF-α and IL-1 . While Orismilast reduces inflammation, it does not significantly reduce the immune system’s ability to fight off infections. For some people, this may make Orismilast more appealing than other anti-inflammatory drugs. Apremilast, a drug similar to Orismilast, has previously been shown to be effective for less severe HS. Iscalimab is a unique injectable drug that blocks a receptor needed to activate various immune system cells including as macrophages and dendritic cells, as well as B-cells. AT193 is a topical medication that reduces the ability of immune cells to produce IL-17. PTM001 is an oral receptor blocker that also influences IL-17 production, but more prominently affects cells’ ability to produce IL-1 as well as TNF-α.
Other Drugs. Gentian violet is an anti-bacterial dye that is cheap and can be obtained over-the-counter (i.e., without a prescription). This topical medicine could be a cost-effective option for patients with HS if it is shown to be effective in clinical trials. Metformin, a drug that is FDA-approved for the treatment of type II diabetes mellitus, has shown promise for patients with HS in small studies. Metformin works by boosting insulin sensitivity and lowering blood sugar, making it a very useful treatment for type II diabetes. Metformin is also thought to reduce inflammatory cytokines that cause disease in HS. A phase III clinical trial is planned to see if metformin could be a good treatment option for patients with HS.
Table 29.2 lists the new non-drug treatments being studied in clinical trials for HS as of January 1, 2023. A detailed review of these treatments is outside the scope of this chapter.
The future of HS treatment appears bright with these promising new therapies being studied in clinical trials, and there is hope that more safe and effective treatment options will become available for patients soon.
VI. Reference articles
- Zouboulis CC, Benhadou F, Byrd AS, et al. What causes hidradenitis supurativa? – 15 years after. Exp Dermatol. 2020;29(12):1154-1170.
- Zouboulis CC, Frew JW, Giamareollos-Bourboulis EJ, et al. Target molecules for future hidradenitis suppurativa treatment. Exp Dermatol. 2021;30 Suppl 1:8-17.
- Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of inflammatory cytokine association in hidradenitis suppurativa. F1000Res. 2018; 7:1930.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81(1):91-101.
- Johann E Gudjonsson JE et al. Contribution of plasma cells and B cells to hidradenitis suppurativa pathogenesis. JCI Insight 2020;5(19):e139930
VII. Questions and Answers
Question 1 How can I find an HS clinical trial in my area? AnswerVisit clinicaltrials.gov. Under the section titled “Find a study”, select the status “Recruiting and not yet recruiting”. Next, in the section labeled “Condition or Disease”, enter “Hidradenitis Suppurativa” and select your country and state. The site allows you to search for clinical trials in specific cities and within a certain distance from your home.
| Drug Name | Route | Target(s) | Sponsor | Phase | Status | |
|---|---|---|---|---|---|---|
| Neutrophils/Complement | ||||||
| LYS006 | Oral | LTA4 hydrolase | Novartis Pharmaceuticals | II | Recruiting | |
| RIST4721* | Oral | CXCR2 receptor | Aristea Therapeutics Inc. | II | Recruiting | |
| BDB-001 | SC | C5a | Staidson (Beijing) Biopharmaceuticals Co., Ltd | I/II | Recruiting | |
| TNF-α | ||||||
| Infliximab biosimilar | SC | TNF-α | Services Institute of Medical Sciences, Pakistan | I | Active, not recruiting | |
| IL-1 | ||||||
| Lutikizumab (ABT-981) | SC | IL-1 | AbbVie | II | Recruiting | |
| MAS825 | SC | IL-1/IL-18 | Novartis Pharmaceuticals | II | Recruiting | |
| IL-36 | ||||||
| Spesolimab | IV, SC | IL-36 receptor | Boehringer Ingelheim | II | Active, not recruiting | |
| Imsidolimab* | IV, SC | IL-36 receptor | AnaptysBio Inc | II | Active, not recruiting | |
| IL-17 | ||||||
| Secukinumab | SC | IL-17 | Novartis Pharmaceuticals | III | Recruiting | |
| Izokibep | SC | IL-17 | Acelyrin Inc. | II | Recruiting | |
| Sonelokimab | SC | IL-17 | Moon Lake Immunotherapeutics AG | II | Recruiting | |
| Bimekizumab | SC | IL-17 | UCB Biopharma SRL | III | Active, not recruiting | |
| JAK | ||||||
| Tofacitinib (for various indications in patients with Down Syndrome) | Oral | JAK 1-3 | University of Colorado, Denver | II | Recruiting | |
| Ruxolitinib 1.5% cream | Topical | JAK 1-2 | Milton S. Hershey Medical Center | II | Recruiting | |
| Incyte Corporation | II | Recruiting | ||||
| Povorcitinib (INCB054707) | Oral | JAK 1 | Incyte Corporation | III | Not yet recruiting | |
| Incyte Corporation | III | Not yet recruiting | ||||
| Multiple Immune System Cells | ||||||
| Fostamatinib | Oral | SYK | Rigel Pharmaceuticals | II | Recruiting | |
| Remibrutinib (LOU064) | Oral | BTK | Novartis Pharmaceuticals | II | Recruiting | |
| Zunsemetinib (ATI-450) | Oral | MK2 | Aclaris Therapeutics Inc. | II | Active, not recruiting | |
| Orismilast | Oral | PDE4 | UNION therapeutics | II | Not yet recruiting | |
| Iscalimab (CFZ 533) | SC | CD40 | Novartis Pharmaceuticals | II | Recruiting | |
| AT193 | Topical | Aryl hydro-carbon receptor | Azora Therapeutics Inc | I | Active, not recruiting | |
| PTM-001 | Oral | P2X7 receptor | Phoenicis Therapeutics | II | Recruiting | |
| Other Meds | ||||||
| Gentian Violet | Topical | Bacteria | Wake Forest University Health Sciences | II | Not yet recruiting | |
| Metformin | Oral | Anti-diabetic | Erasmus Medical Center | III | Unknown |
Phase I-III “clinical” trials registered with clinicaltrials.gov for hidradenitis suppurativa that were either active, recruiting or not yet recruiting as of January 1, 2023.
Abbreviations; CXCR, CXC chemokine receptor; C5a, complement 5a; LTA4, leukotriene A4; IL, interleukin; IV, intravenous; JAK, Janus Kinase; SC, subcutaneous; PDE4, phosphodiesterase 4; TNF, tumor necrosis factor; MK2, p38MAPK-activated protein kinase 2; SYK, spleen tyrosine kinase
* While still in clinical trials as of January 1, 2023, at the time of publication these drugs were no longer in the pipeline for HS treatment. The trial for RIST4721 was stopped due to safety concerns. A press release from the company for Imsidolimab stated that the results from their phase II clinical trial did not show that it was effective for HS and the company will be stopping Imsidolimab’s development for HS.
| Name | Sponsor | Status | |
|---|---|---|---|
| Procedures | |||
| Alexandrite Laser | Wayne State University | Active, not recruiting | |
| Axillary deroofing followed by serial 1064nm Nd:YAG laser treatments | University of Texas at Austin | Not yet recruiting | |
| Axillary perforator flap versus secondary wound healing | Assistance Publique – Hôpitaux de Paris | Not yet recruiting | |
| Botulinum toxin | University of Pittsburg | Recruiting | |
| Fecal microbiota transplantation for various indications | Odense University Hospital | Not yet recruiting | |
| Fractional ablative 2940nm Er:YAG laser for HS scarring | Montefiore Medical Center | Not yet recruiting | |
| Fractional ablative carbon dioxide laser with topical steroids | University of Southern California | Not yet recruiting | |
| Intralesional diode laser | Zealand University Hospital | Recruiting | |
| Wound Care | |||
| Methylene blue, gentian violet, and bovine forestomach | Wake Forest University Health Sciences | Not yet recruiting | |
| NovaSorb® biodegradable temporizing matrix versus human allograft | Joseph M. Still Research Foundation, Inc | Not yet recruiting | |
| Observational registry for use of Myriad Matrix™ and Myriad Morcells™ in soft tissue reconstruction for various indications | Aroa Biosurgery Limited | Recruiting | |
| Petrolatum w/ non-stick bandage vs wet to-dry dressing post HS | University of North Carolina, Chapel Hill | Recruiting | |
| Procellera bioelectric dressing vs gauze dressing post deroofing | University of Miami | Recruiting | |
| CAM | |||
| Battlefield Acupuncture | Wayne State University | Recruiting | |
| Mindfulness Training | University of Miami | Not yet recruiting |
Phase I-III clinical trials registered with clinicaltrials.gov for hidradenitis suppurativa that were either active, recruiting or not yet recruiting as of January 1, 2023.
Abbreviations. Er:YAG, erbium-doped yttrium aluminium garnet laser; Nd-YAG, neodymium-doped yttrium aluminum garnet; nm, nanometer.